1. AMINO ACIDS AND PEPTIDES
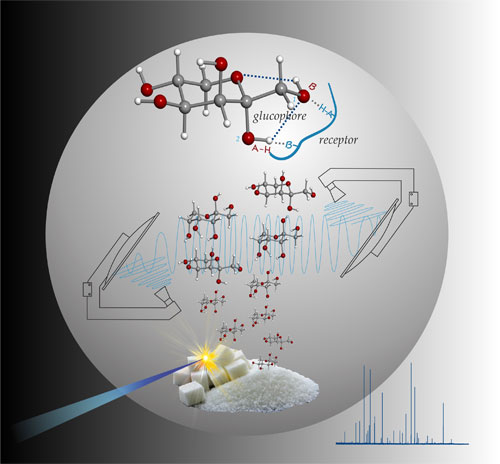
The conformational behaviour of amino acids is of critical interest to understand the dynamical role of these molecules in protein or polypeptide formation. Consequently, extensive structural research has been conducted on amino acids in their natural solid-state phase. However, in the solid state amino acids present a zwitterionic structure (i.e. a bipolar, ionized form of the type +H3N-CH(R)-COO-), which does not occur in the polypeptide chain. In peptides and proteins amino acids participate in their neutral forms. To obtain the structure of the neutral amino acids research should be conducted in the gas phase, in an atmosphere that is essentially free of intermolecular interactions with other partners. In particular, the collisionless environment of a supersonic jet seems particularly well suited for such studies. Rotational spectroscopy is the only technique that, thanks to its inherently superior resolution, can distinguish unambiguously between different isomers, conformers, or isotopomers and provide accurate structural information directly comparable to the in vacuo theoretical predictions.
However, amino acids are difficult to vaporize due to their high melting points and thermal unstability. The analysis of the rotational spectrum of natural amino acids started in the late 1970s on glycine and alanine, but it was refrained for a long time by the difficulties to bring these compounds into gas phase.
We have recently impulsed this field with the combination of laser ablation with Fourier transform microwave spectroscopy in supersonic jets. Over the last years, we have studied the gas-phase conformational behaviour and structure of 18 natural amino acids (histidine, glutamic acid, asparagine,…) and non natural (AC3C, BAIBA, …), to understand the role of intramolecular hydrogen bond interactions in the stabilization of the preferred conformations.
Featured work
Tautomerism in Neutral Histidine, Angew.
Chem. Int. Ed. 2014, 53, 11015-11018
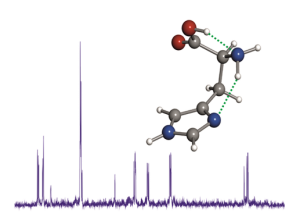
Histidine is an important natural amino acid, involved in many relevant biological processes, which, because of its physical properties, proved difficult to characterize experimentally in its neutral form. In this work, neutral histidine has been generated in the gas phase by laser ablation of solid samples and its NεH tautomeric form unraveled through its rotational spectrum. The quadrupole hyperfine structure, arising from the existing three 14N nuclei, constituted a site-specifically probe for revealing the tautomeric form as well as the side chain configuration of this proteogenic amino acid.
Last contributions
*Fourier transform microwave spectroscopy of Ac-Ser-NH2:
the role of the side chain interactions in peptide folding, Phys.
Chem. Chem. Phys., 2015, 17, 20274-20280
*The conformational locking of asparagine,
Chem. Commun. 2012, 48, 5934
*Preferred Conformers of Proteinogenic Glutamic Acid, J. Am.
Chem. Soc. 2012, 134, 2305-2312
2. CARBOHYDRATES
Carbohydrates are one of the most versatile biochemical building blocks, widely acting in energetic, structural, or recognition processes. The importance of its structure has been the driving force behind the development of methods for elucidating the shape of their building blocks, monosaccharides. Thus, it comes as no surprise that 3D structures and relative stability of conformers of monosaccharides continue to be an area of great research interest. The subtle variation in hydroxyl arrangement is thought to account for differences in chemical and physical properties of the sugars. This is also relevant to distinguish between different conformers. Additionally, monosaccharides are also of interest in the field of astrophysics. The availability of rotational data has been the main bottleneck for examining the presence of these building blocks in the interstellar medium (ISM). Based on the rotational spectra identification, the simplest C2 sugar of glycolaldehyde has been identified, but has yet to detect the C3 sugar of glyceraldehyde. The experimental results obtained in condensed phases seem to indicate that a subtle balance between intrinsic and environmental effects governs the conformational preferences of monosaccharides;
Featured work
Sweet Structural Signatures Unveiled in Ketohexoses
C. Bermúdez, I. Peña, S. Mata, J. L. Alonso
Chem. Eur. J. 2016, 22, accepted (COVER)
The conformational behaviour of naturally occurring ketohexoses has been revealed in a supersonic expansion by Fourier transform microwave spectroscopy coupled with a laser ablation source. Three, two and one conformers of D-tagatose, D-psicose and L-sorbose, respectively, have been identified by their rotational constants extracted from the analysis of the spectra.
Singular structural signatures involving the hydroxyl groups OH(1) and OH(2) have been disentangled from the intricate intramolecular hydrogen bond networks stabilising the most abundant conformers. The present results place the old Shallenberg and Kier sweetness theories on a firmer footing.
Structural expression of exo-anomeric effect
Phys. Chem. Lett., 2016, 7, 845-850
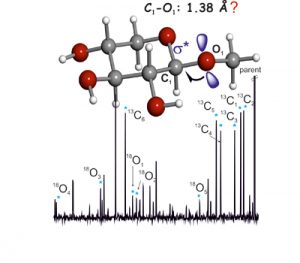
Structural signatures for exo-anomeric effect have been extracted from the archetypal methyl-β-D-xyloside using broadband Fourier transform microwave spectroscopy combined with laser ablation. Spectrum analysis allows the determination of a set of rotational constants, which has been unequivocally attributed to conformer cc-β-4C1 g−, corresponding to the global minimum of the potential energy surface, where the aglycon residue (CH3) orientation contributes to maximization of the exo-anomeric effect. Further analysis allowed the determination of the rs structure, based on the detection of 11 isotopologues-derived from the presence of six 13C and five 18O atoms-observed in their natural abundances. The observed glycosidic C1−O1 bond length decrease (1.38 Å) can be interpreted in terms of the exo-anomeric effect. As such, the exo-anomeric effect presents itself as one of the main driving forces controlling the shape of many biologically important oligosaccharides.
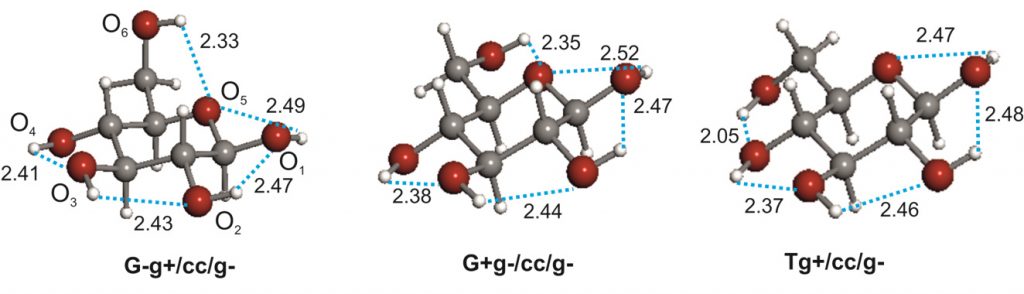
The conformational behaviour of free D-glucose-at last,
Chem. Sci., 2014, 5,515-522
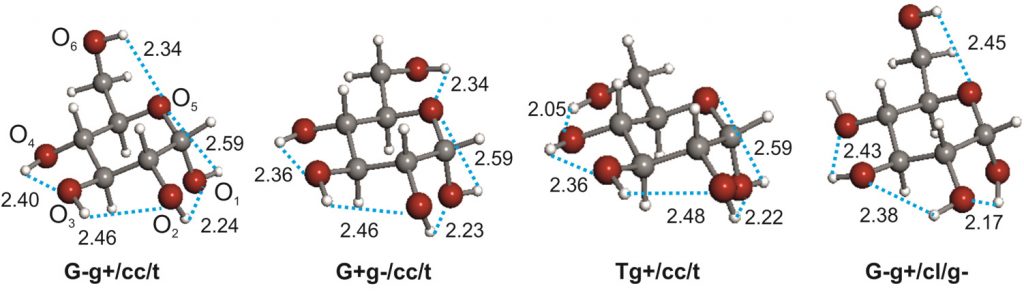
The conformational behaviour of isolated D-glucose has been revealed in this work using Fourier transform microwave spectroscopy coupled with laser ablation of crystalline α- and β-glucopyranose samples. Four conformers of α-D-glucopyranose and three of β-D-glucopyranose have been unequivocally identified on the basis of the spectroscopic rotational parameters in conjunction with ab initio predictions.
Stereoelectronic hyperconjugative factors, like those associated with anomeric or gauche effects, as well as the cooperative OH O chains extended along the entire molecule, are the main factors driving the conformational behaviour. The most abundant conformers exhibit a counter-clockwise arrangement (cc) of the network of intramolecular hydrogen bonds.
O chains extended along the entire molecule, are the main factors driving the conformational behaviour. The most abundant conformers exhibit a counter-clockwise arrangement (cc) of the network of intramolecular hydrogen bonds.
α-D Glucose
β-D Glucose
Last contributions
*The shape of D-glucosamine,
Phys. Chem. Chem. Phys., 2014, 16, 23244-23250
*Erythrose revealed as furanose forms,
Chem.Commun., 2013, 49, 10826-10828
*Six Pyranoside Forms of Free 2-Deoxy-D-ribose,
Angew. Chem. Int. Ed., 2013, 52, 11840-11845
*Conformations of D-xylose: the pivotal role of the intramolecular hydrogen-bonding,
Phys. Chem. Chem. Phys., 2013, 15, 18243-18248
*Unveiling the Sweet Conformations of D-Fructopyranose,
ChemPhysChem 2013, 14, 893-895
3. NEUROTRANSMITTERS AND DRUGS
Combining molecular-beam Fourier transform microwave (MB-FTMW) spectroscopy with heating methods, we have characterized the conformational panoramas of several neurotransmitters in the gas phase, including 2-phenylethylamine, p-methoxyphenylethylamine, norephedrine, ephedrine, and pseudoephedrine. However, the investigation of a complete series of neurotransmitters by MB-FTMW spectroscopy is not possible because of the high melting points and associated very low vapor pressures of these compounds. Laser ablation in combination with molecular beams and microwave spectroscopy (LA-MB-FTMW spectroscopy)has proven to be a powerful tool in the investigation of the gas-phase conformational behavior of solid neurotransmitters such as taurine, tryptamine,γ-aminobutyric acid (GABA),serotonin,dopamine, octopamine and synephrine.
Featured works
Seven Conformers of Neutral Dopamine Revealed in the Gas Phase,
J. Phys. Chem. Lett., 2013, 4, 486-490
The rotational spectrum of neutral dopamine has been investigated for the first time using a combination of Fourier transform microwave spectroscopy with laser ablation. The parameters extracted from the analysis of the spectrum unequivocally identify the existence of seven conformers of dopamine. 14N nuclear quadrupole coupling interactions have been used to determine the orientation of the amino group probing the existence of stabilizing N–H···π interactions for all observed conformers.
Analysis of illicit drugs by direct ablation of solid samples
Eur. J. Mass Spectrom. 2015, 21, 775–781
Analysis of illicit drugs arises as an important field of work given the high social impacts presented by drugs in the modern society. Direct laser ablation of solid compounds allows their analysis without sampling or preparation procedures. For that purpose, an experimental set-up that combines laser ablation with time-of-flight mass spectrometry has been constructed very recently to perform studies on the mass spectra of such drugs as 3,4-methylenedioxy-N-methylamphetamine, commonly known as MDMA or ecstasy. Analysis of the observed fragmentation pattern in mass spectra may elucidate the ablation-induced photofragmentation phenomena produced, which differ from those previously observed with conventional ionization methods.
Last contributions
*Conformational Analysis of Octopamine and Synephrine in the Gas Phase,
J. Phys. Chem. A, 2013, 117, 4907-4915
*The microwave spectrum of neurotransmitter serotonin,
Phys. Chem. Chem. Phys. 2012, 14, 13618-13623
*Conformational behaviour of norephedrine, ephedrine and pseudoephedrine,
J. Am. Chem. Soc., 2009, 131, 4320-4326
4. NUCLEIC ACID BASES
Featured work
Angew. Chem. 2013, 125, 2387-2390
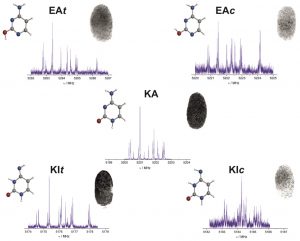
Give me five! All five tautomers and conformers of cytosine were characterized in the gas phase by laser ablation molecular beam Fourier transform microwave spectroscopy. The spectra were assigned unambiguously on the basis of the hyperfine structure due to the three 14N nuclei (see picture; N blue, O red). The relative energies of the identified species were estimated from the relative intensities of the spectra.
Last contributions
*Accurate molecular structure and spectroscopic properties of nucleobases: a combined
computational–microwave investigation of 2-thiouracil as a case study,
Phys. Chem. Chem. Phys., 2013, 15, 16965-16975
*Rotational spectral signatures of four tautomers of guanine,
Angew. Chem. Int. Ed., 2009, 48, 6141-6143
*Probing thymine with Laser Ablation Molecular Beam Fourier Transform microwave spectroscopy,
J. Chem. Phys., 2007, 126, 191103
*The structure of uracil: A Laser Ablation rotational study,
J. Phys. Chem. A, 2007, 111, 3443-3445
5. NUCLEOSIDES
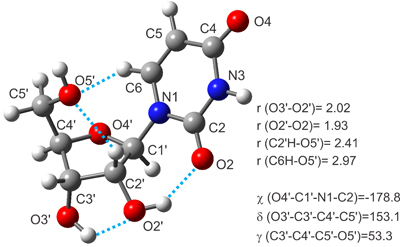
The nucleoside uridine has been placed in the gas phase by laser ablation and characterized by Fourier transform (FT) microwave techniques. Free from the bulk effects of their native environments, anti/C2’-endo-g+ conformation has been revealed as the most stable form of uridine. Intramolecular hydrogen bonds involving uracil and ribose moieties have been found to play an important role in the stabilization of the nucleoside.
Featured work
The nucleoside uridine isolated in the gas phase
Angew. Chem. Int. Ed. 2015, 54, 2991–2994 (COVER)